Marketing authorisation procedures in Europe
24.6.2020
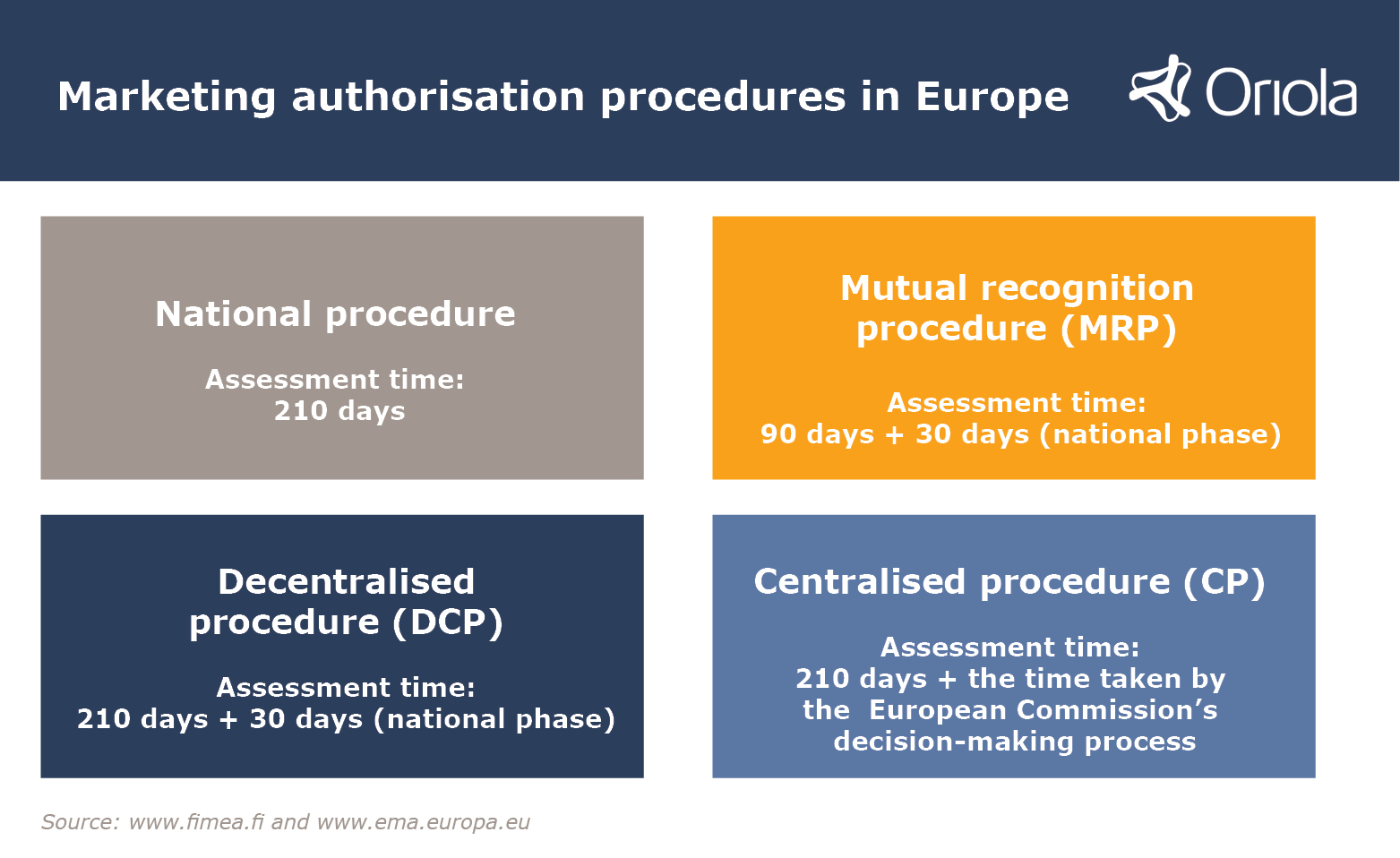
In order for a medicinal product to be available on the market, sold and marketed, it needs to have a marketing authorisation. The marketing authorisation application must provide evidence of the efficacy, safety and quality of the medicine. When applying for a marketing authorisation for a medicine, the marketing authorisation application is submitted to a regulatory authority, which will assess the medicinal product’s pharmaceutical and chemical quality, efficacy and safety, as well as its risk-benefit ratio. In the EU, there are four types of marketing authorisation application procedures. We have compiled a handy checklist for you of the ways in which these procedures differ from each other.
If you are facing challenges in regulatory affairs, our team of experts is at your service!
National procedure
- Used when applying for a marketing authorisation in one individual EU member state, Norway or Iceland
- The national procedure can only be used if the medicinal product does not already have a marketing authorisation in another EU member state, Norway or Iceland.
- Assessment time: 210 days.
Mutual recognition procedure (MRP)
- The mutual recognition procedure should be used if the product already has a marketing authorisation in another EU -member state, Norway or Iceland. The country in which the national marketing authorisation has been granted acts as the reference member state, and the other countries concerned recognise the marketing authorisation.
- Assessment time: 90 days + 30 days (national phase).
Decentralised procedure (DCP)
- The decentralised procedure should be used if the product does not yet have a marketing authorisation in any EU country, Norway or Iceland, and if the applicant would like to apply for a marketing authorisation in several countries at once.
- In the decentralised procedure, the applicant asks one member state to act as the reference member state which evaluates the application and prepares an assessment report. The other member states can comment on the assessment report. At the end of the process, a marketing authorisation will be granted in each of the involved member state after completion of the national phase.
- Assessment time: 210 days + 30 days (national phase).
Centralised procedure (CP)
- Using the centralised procedure means applying for a single marketing authorisation that covers all EU countries, Norway and Iceland.
- In the centralised procedure, marketing authorisation applications are assessed by the European Medicines Agency (EMA), and the marketing authorisation is granted by the European Commission.
- The centralised procedure is primarily used for new active substances, and it is mandatory for biopharmaceuticals and other innovative medical products.
- Assessment time: 210 days + the time taken by the European Commission’s decision-making process.
Read more about our regulatory affairs services here or contact us for more information.
Source: www.fimea.fi and www.ema.europa.eu

Laura Riihimäki-Lampén
Head of Regulatory Affairs and Translations +358 50 409 9161 laura.riihimaki-lampen@oriola.com


